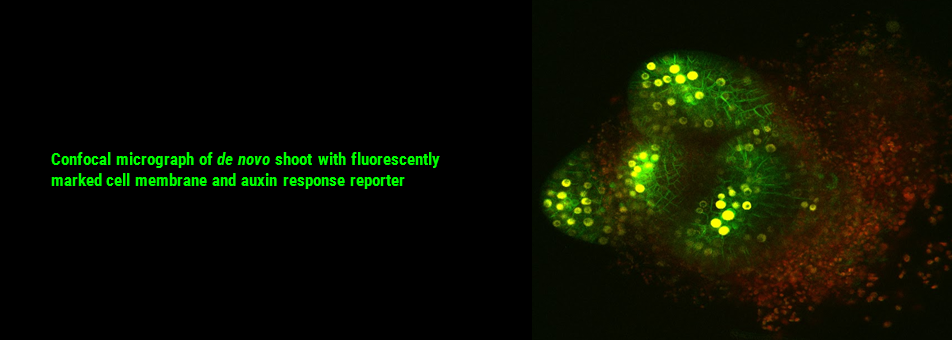
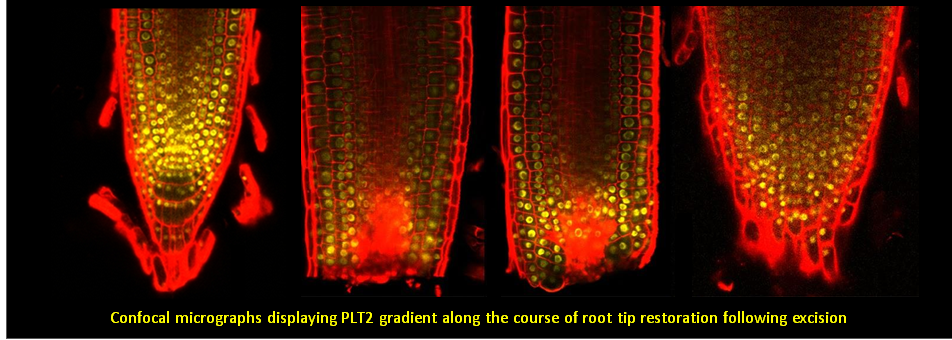
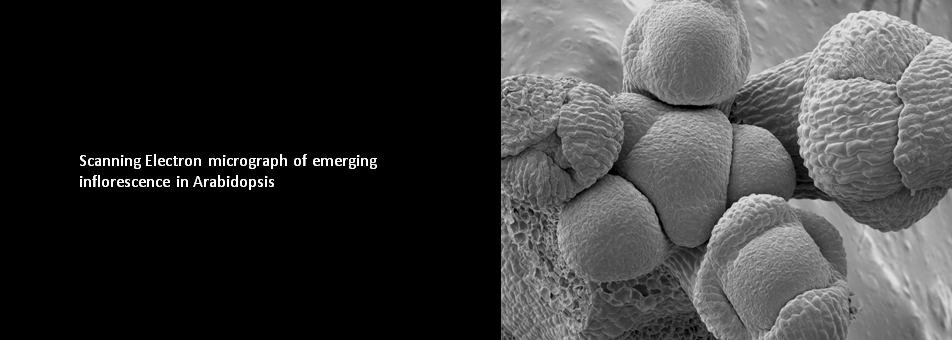
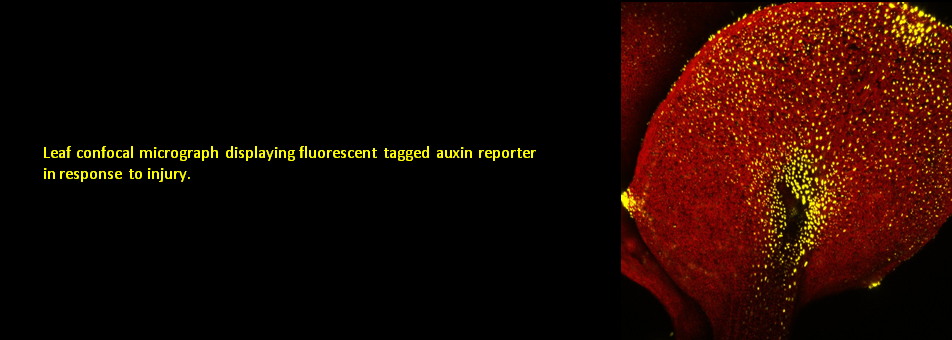
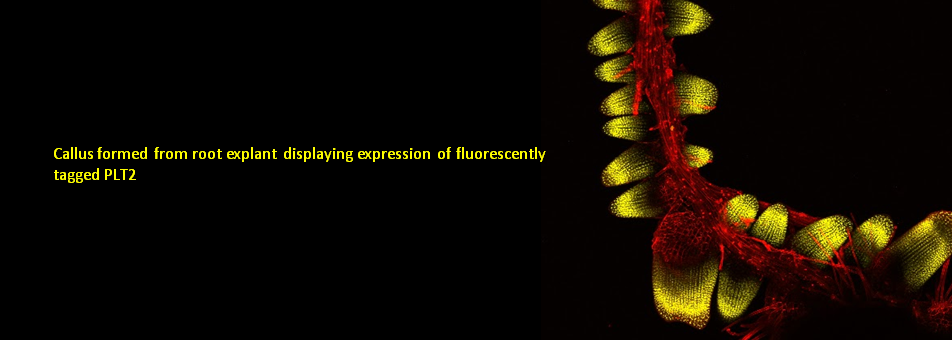
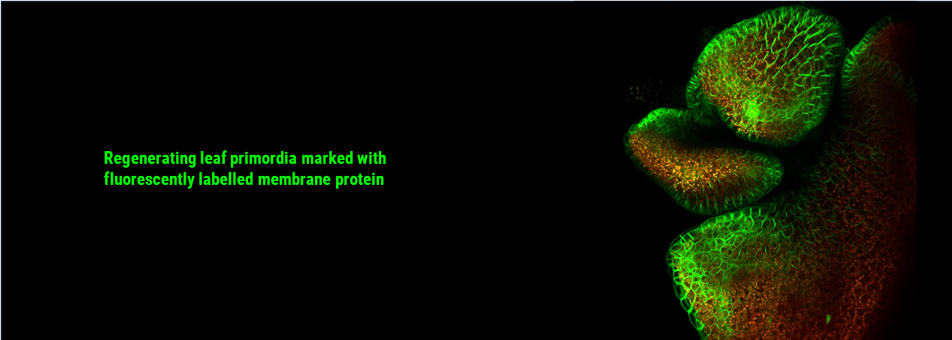
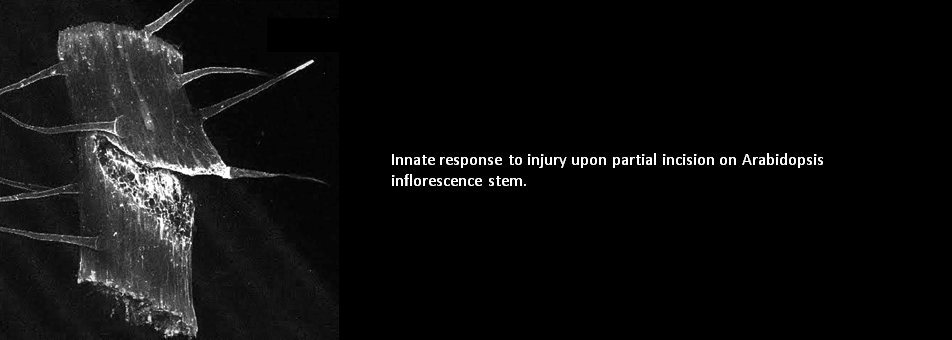
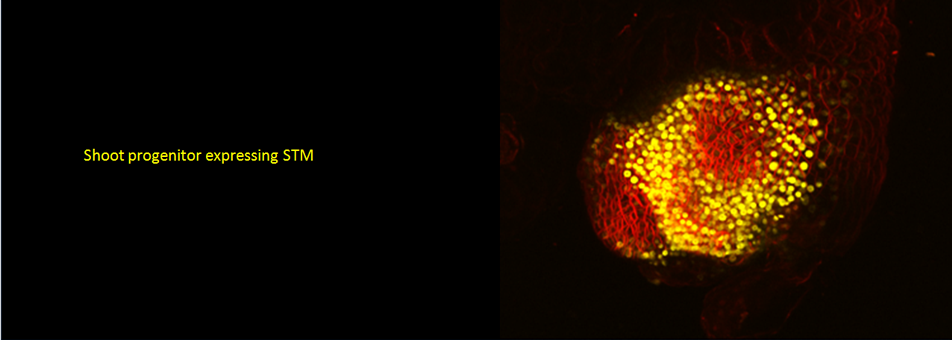
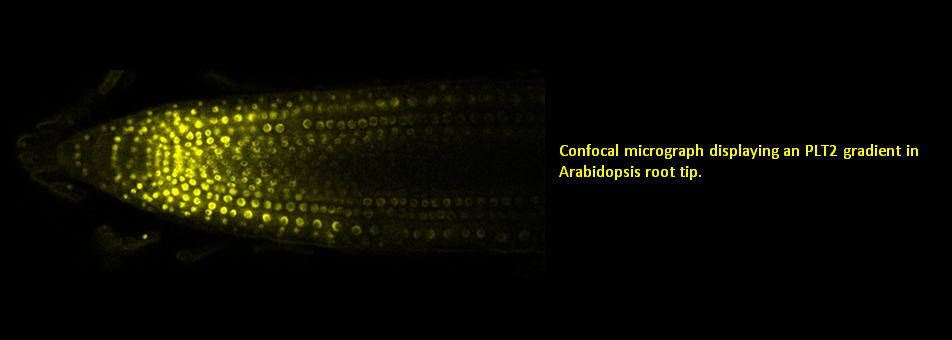
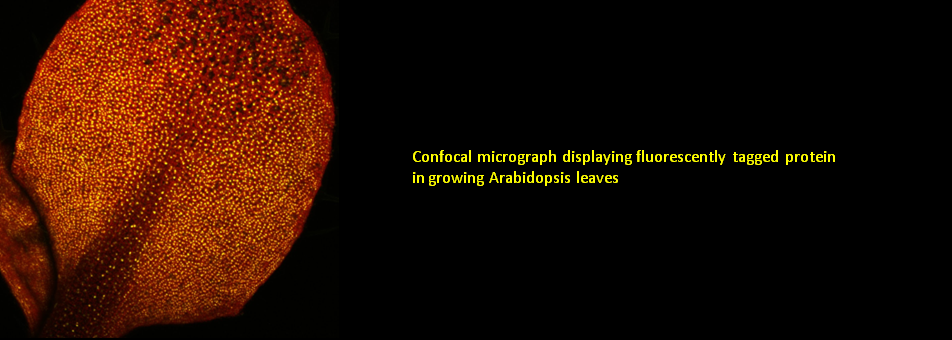
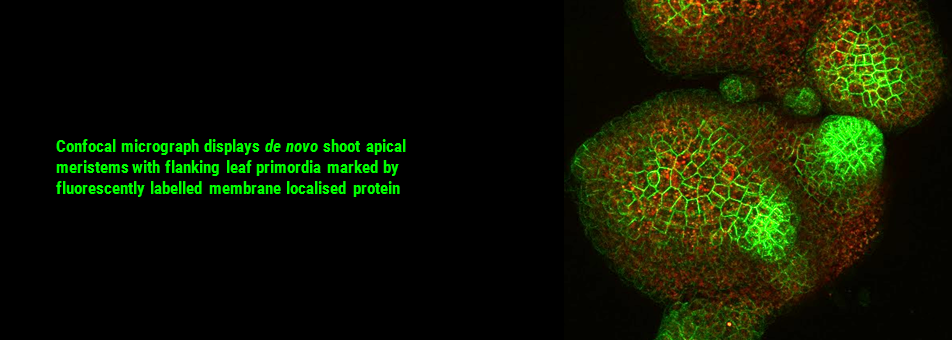
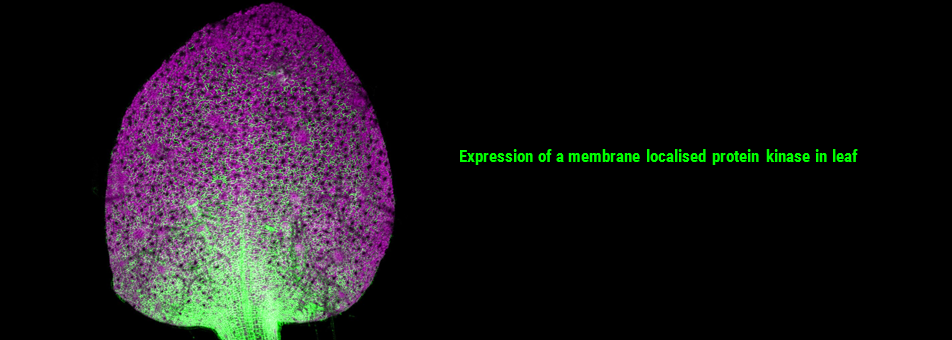
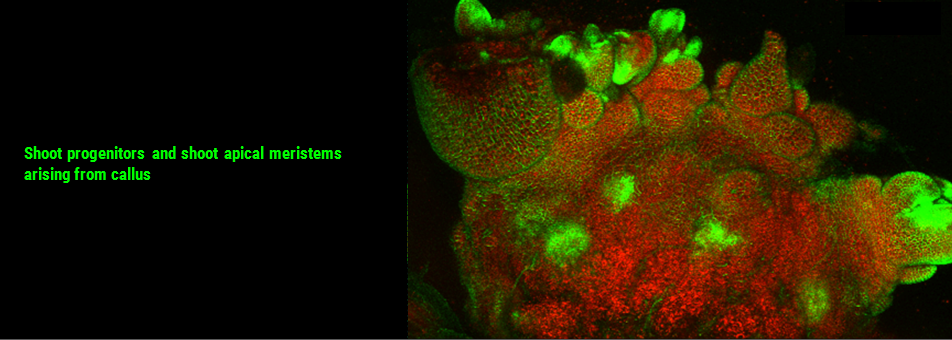
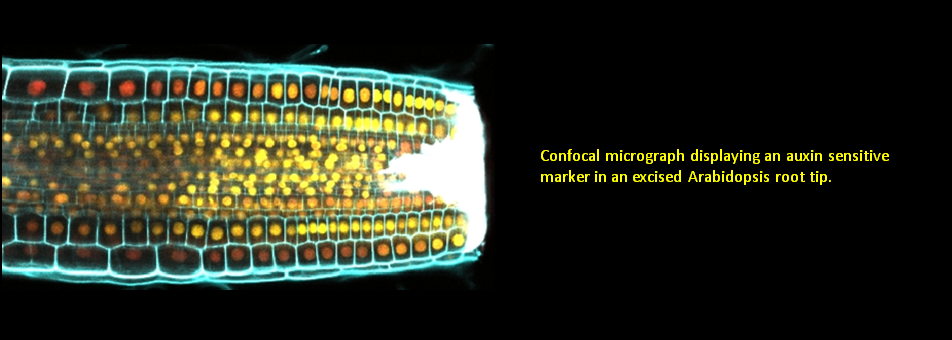
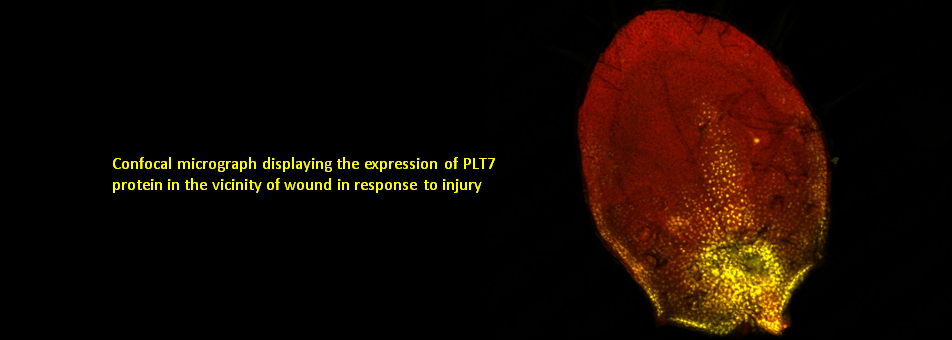
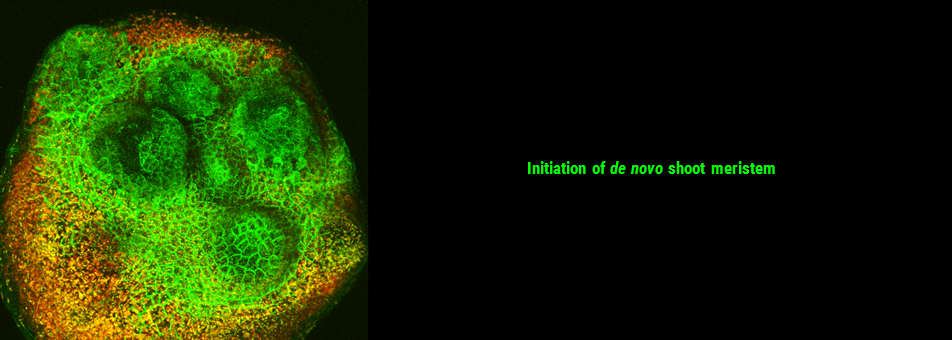
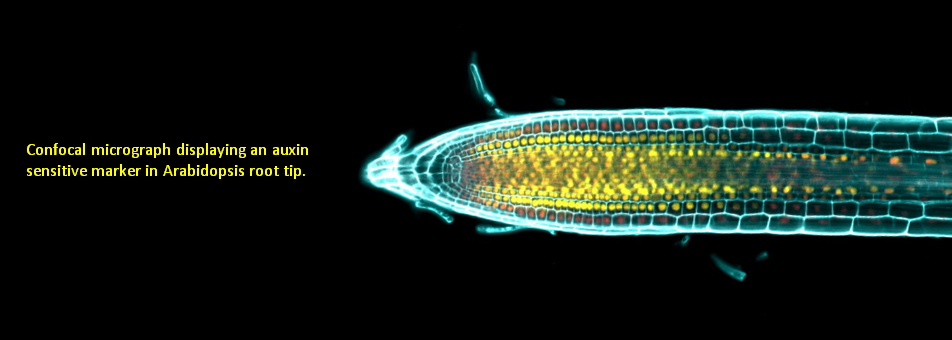
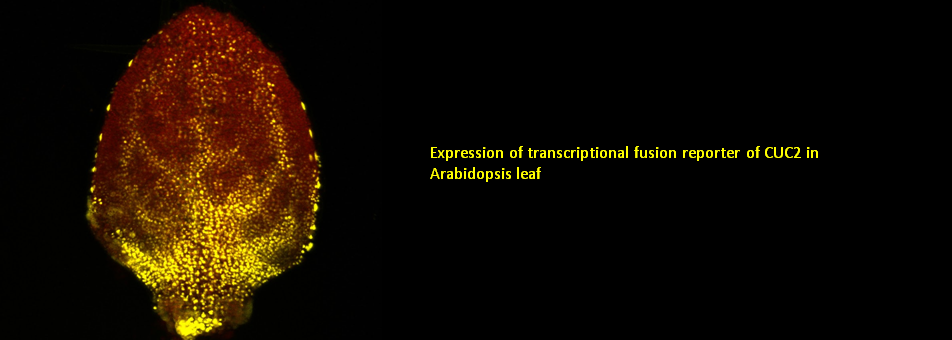
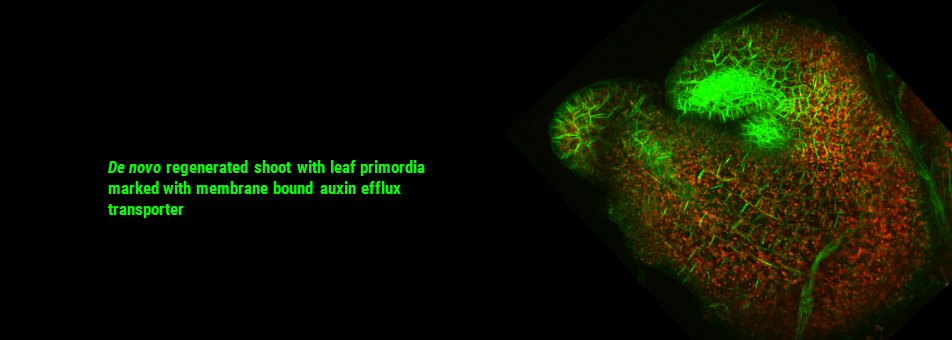
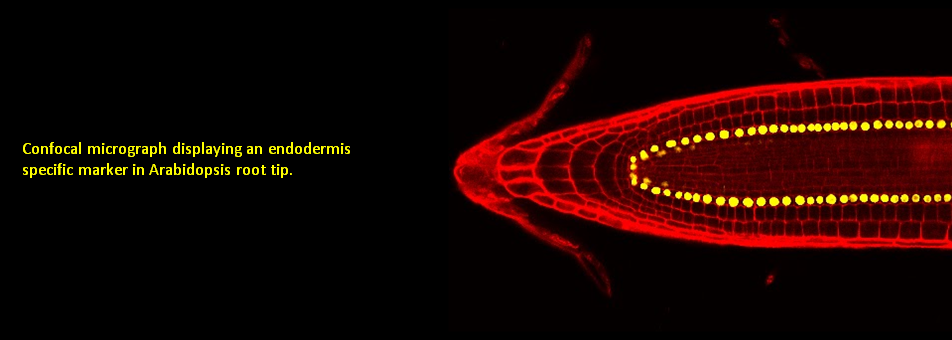
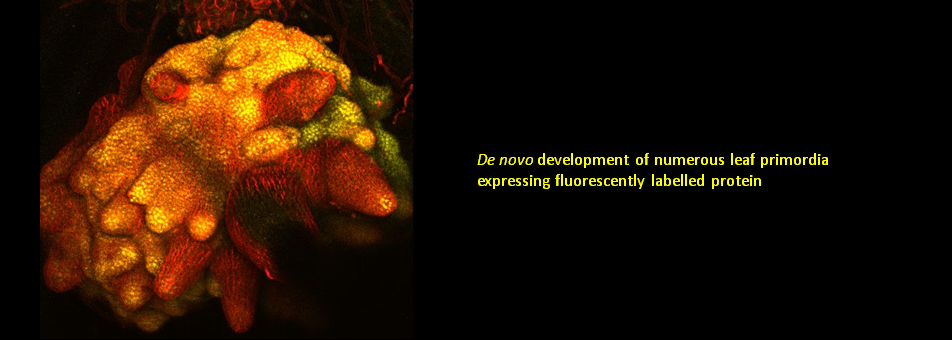
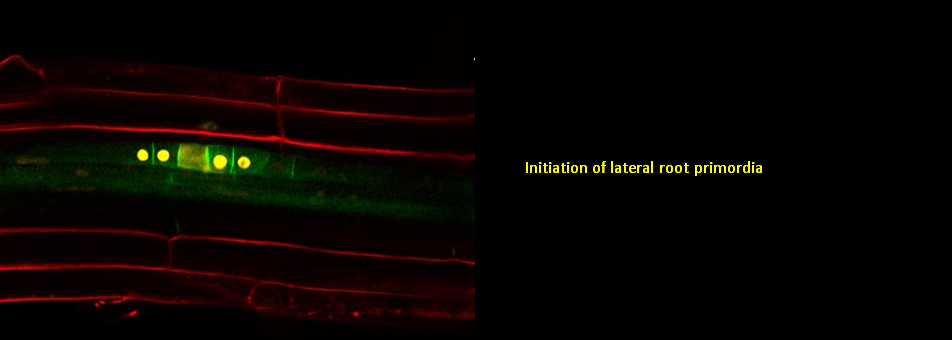
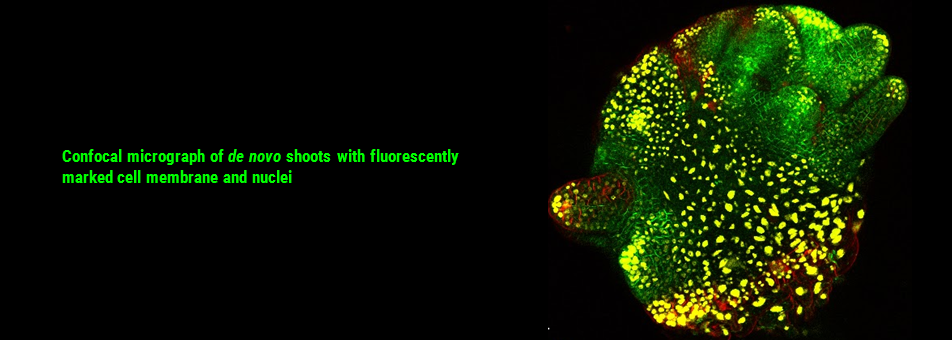
We use Arabidopsis as model to study plant regeneration.
To learn more about this please read
To learn more about de novo shoot regeneration please read following articles.
• Mechanical conflict caused by a cell-wall-loosening enzyme activates de novo shoot regeneration. (https://www.sciencedirect.com/science/article/pii/S1534580722005482)
• Shoot meristem progenitors emerge from mechanical heterogeneities. (https://doi.org/10.1016/j.devcel.2022.08.004)
To learn more about regeneration of tissues or organs which were lost in injury, please read the following papers.